My research interests center around the so-called lipophilic pigments. Specifically, these are the chlorophylls, chlorophyll derivatives, carotenoids, certain polyaromatic hydrocarbons (perylene) and a dimeric indole-phenol (scytonemin).
An examination of my vita reveals that I have had certain periods of research. The early seventies included studies on de novo carotenogenesis in various species of the genus Mycobacterium. Several of the mycobacteria are photochromogenic, producing pigments only when induced to do so by light – and light of the proper wavelength. This was my introduction into the fascinating world of biological pigments. I extended my interest in carotenoids with my master’s thesis topic – carotenoids metabolism in Crustacea, namely the blue-crab (Callinectes sapidus Rathbun 1895). From the late seventies through the early nineties, I was involved in organic geochemical studies of deep sea, lake and coastal sediments as well as sediment traps and selected experimental (senescence / death) studies. These investigations traced chlorophyll and its derivatives from living biota into the geosphere to eventual destruction (recycle) or preservation as a variety of free-base and metallo- geoporphyrins, notably in oil shales and petroleum crudes. For most of the past decade I have been involved in two main aspects of the biogeochemistry of chlorophylls and carotenoids, namely the changes that occur during the senescence / death of microalgal cells and the use of pigment distributions as “chemotaxonomic” markers for the assessment of microalgal community structure, productivity and overall dynamics. Most of these studies deal directly or indirectly with the ecosystems affected by the Comprehensive Everglades Research Plan (CERP).
Following I discuss a few points pertinent to my present research efforts. I will be glad to delve deeper into any portion of my studies and potential collaborative research, especially with prospective students in the Department of Chemistry and Biochemistry and the Environmental Sciences Program.
Pigment-based chemotaxonomy, algal blooms & community dynamics:
The chlorophylls are the main pigments of photosynthesis. (see http://life.uiuc.edu/govindjee/paper/gov.html or http://photoscience.la.asu.edu/photosyn) They process trapped solar energy into ‘reductant’ (NADPH, e-), which can then reduce (‘fix’) the carbon in carbon dioxide (CO2) to organic carbon. This is the so-called carbon fixation which yields the organic compounds (sugars, proteins, fats etc.) fuels and builds the majority of life on earth.
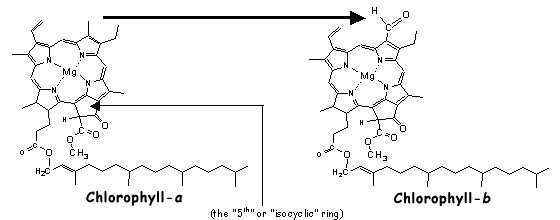
The most common, abundant and best-studied chlorophyll is chlorophyll-a, as shown here.

Chlorophyll-a can either be part of the solar energy-gathering complex or serve as the hinge point for the conversion of that energy to chemical energy for carbon fixation. That is, special chlorophyll-a molecules exist in the so-called antenna array. Non-antenna chlorophyll-a molecules plus a wide variety of ‘accessory’ pigments pass ‘solar energy’, as electron flow to the antenna pigments where photosynthesis is initiated.
Throughout the history of life on Earth, numerous ‘accessory’ pigments have evolved. These accessory pigments are able to either absorb parts of the electromagnetic spectrum (~ light for our purposes) which chlorophyll-a cannot and/or do so in a more efficient manner. A single chemical change in chlorophyll-a, the conversion of a methyl to formyl group (Fig.R1, arrow), gives chlorophyll-b, an accessory pigment in green algae (Chlorophyta) and all higher plants.
Photoautotrophs that use water (H2O) to generate the reductants (NADPà NADPH) yield molecular oxygen (O2) as a by-product. This is called oxygenic photosynthesis. The presence of chlorophyll-a reveals the existence of >oxygenic photosynthesis and, even though it can be related (‘estimated’) to overall photosynthetic biomass, it tells us nothing about the taxonomic makeup of that community. Photoautotrophs that utilize hydrogen sulfide (H2S) as a source of reductant, giving various forms of sulfur (S8o, etc.) as by products, are involved in anoxygenic photosynthesis. These anoxygenic species contain a variety of bacteriochlorophylls (Fig.R2; -a in purple-S bacteria, -c/-d/-e/-f/-g in the green and brown sulfur bacteria).
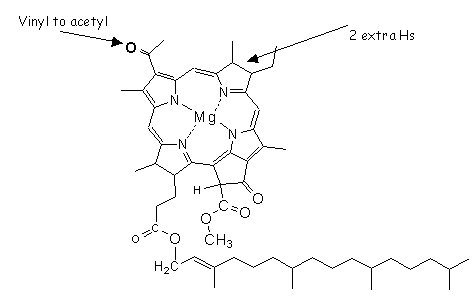
Thus, the presence and abundance of chlorophyll-a and the bacterio-chlorophylls can be utilized to estimate the relative importance of oxygenic and anoxygenic photoautotrophs and their organic matter (e.g. protein biomass) in an ecosystem.
Likewise, one can determine the presence and estimate the abundance of the various taxonomic groups of the oxygenic photoautotrophs through total pigment analysis.
As stated, there are several photosynthetic accessory pigments (PAPs). This group includes chlorophyll-b (chlorophytes, higher plants), fucoxanthin plus the chlorophylls-c (chrysophytes, diatoms and relatives), gyroxanthin diester (Florida Red Tide, Karenia brevis) peridinin (dinoflagellates), and the divinyl chlorophylls-a / -b (prochlorophytes). Additionally, there are many taxon specific (or abundant, “zea”) photoprotectorant pigments (PPPs), such as zeaxanthin (“zea”, cyanobacteria), myxoxanthophyll (cyanobacteria), keto-carotenoids (echinenone, canthaxanthin: cyanobacteria), lutein (chlorophytes), alloxanthin (cryptophytes), and others. The structures of but a few of these are given below.
Zeaxanthin (cyanobacteria and a ‘bit’ in Chlorophytes)
Fucoxanthin (Chrysophytes)
19’-butanoyloxy- and 19’-hexanoyloxy-fucoxanthins ( prymnesiophytes)
Peridinin (Pyrrhophyta, Dinoflagellates)
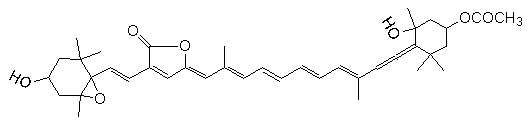
Gyroxanthin diester (Florida Red Tide, Karenia brevis: Pyrrhophyta)
Therefore, through pigment analysis and the relation of the abundance of these ‘biomarkers’ to chlorophyll-a, one is able to estimate the (chemo-) taxonomic structure of microalgal communities. This methodology, pigment-based chemotaxonomy, has gained increasing favor for rapid temporal and spatial investigations of microalgal communities, such as phytoplankton distributions in lakes and oceans. The main premise rests with our ability to assign a numerical relationship between the marker pigment and chlorophyll-a, the biomass marker. As an example, the analyses of a great many diatoms reveals that the chlorophyll-a to fucoxanthin (molar) ratio is 1.1 : 1. Hence, for every mole of fucoxanthin that we find in a sample, we may derive 1.1 moles of diatom-contributed chlorophyll-a. This same process is then extended to all of the marker pigments that we isolate and identify from a sample. The percent composition of the community is then calculated by the relative abundances of the taxon-specific chlorophyll-a.
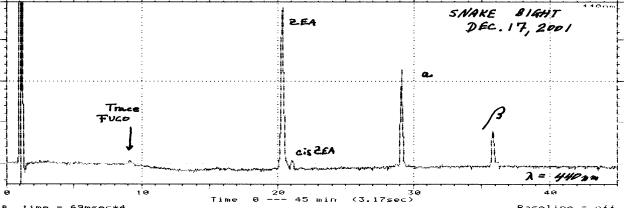
An example of the pigment analysis of a very simple phytoplankton community (cyanobacteria / diatom ~95% / ~ 5%) is given below (Fig. R4). This sample, from Snake Bight in north-central Florida Bay was undergoing a cyanobacterial bloom (Total CHL-a = 13.3 mg / L) at the time of collection. The great dominance of cyanobacteria was indicated by the abundance of zeaxanthin and only a trace of fucoxanthin (diatoms) could be found.
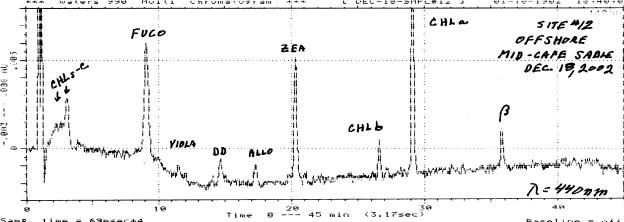
A more complicated, but also more interesting, pigment distribution is shown as Figure R5. Here, a mixed community (~ 21/20/44/0/15 % as cyanobacteria / chlorophytes / diatoms / dinoflagellates / cryptophytes) was indicated by the relative abundances of zeaxanthin, chlorophyll-b, fucoxanthin, peridinin (absent) and alloxanthin, respectively (Total CHL-a ~ 1.5 mg / L).
Open ocean deep water phytoplankton assemblages, such as the example below (Fig. R6), often contain a mixture of prymnesiophytes and prochlorophytes. These taxa are detected based upon the presence of the 19’-butanoyloxy- and 19’-hexanoyloxy-fucoxanthins or divinyl chlorophyll-a, respectively.
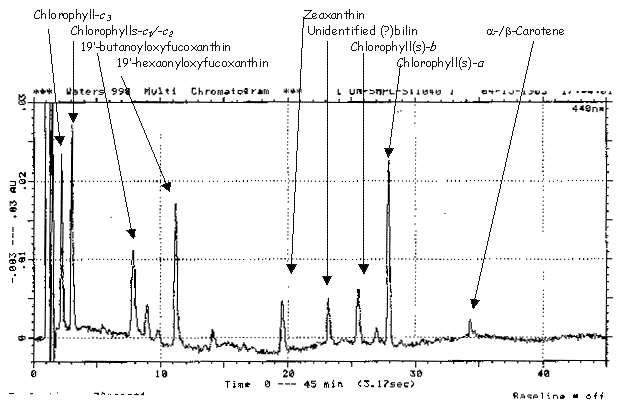
As you may have noticed, I mentioned 2 forms of chlorophyll-a yet only 1 is seen in the C-18 RP-HPLC chromatogram (Fig.R6) above. This is because C-18 columns fail to resolve ‘regular’ (MonoVinyl “MV”) CHLa from divinylchlorophyll-a (DV-CHLa). However, by running these pigment extracts in C-8 reverse phase columns (Fig. R7), we can easily separate MV and DV CHL-a.
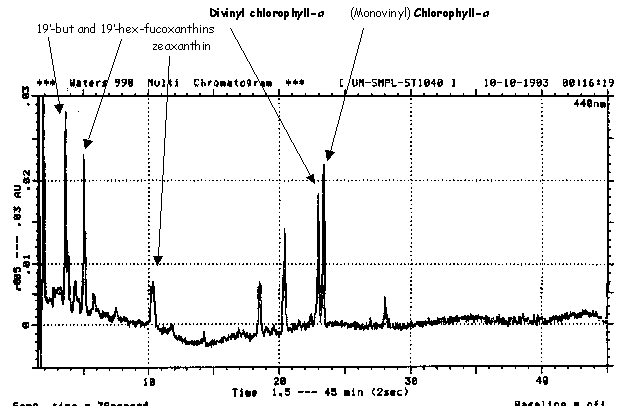
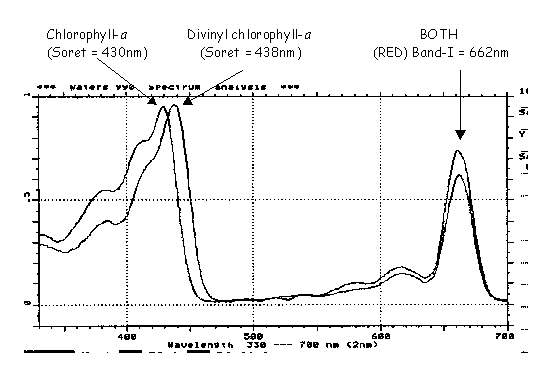
Aside from chromatographic retention time, the first dimension of analytical identification, all ‘peaks’ are also analyzed for their absorption spectrum using full spectral (190-800 nm) photodiode array or ‘PDA’ detection. In this manner, the difference between divinyl and ‘regular’ (monovinyl) chlorophyll-a is easily noted (Fig. R8).
Compared to chlorophyll-a (Fig. R8: maxima at 430 and 662 nm), divinyl chlorophyll-a (maxima at 438 and 662 nm) exhibits an 8 nm red shift in the larger, so-called Soret, absorption band. Thus, the 2 dimensional identification of retention time, on C-8 columns) and the absorption spectrum allows for the identification of DV-CHLa and, by chemotaxonomic principles, the Prochlorophyta.
Pigment-based chemotaxonomy, while it will never replace microscopic examination by a highly skilled taxonomic expert, is extremely useful in the rapid spatial and temporal monitoring of ecosystems. That is, the microscopist can take identification to the species level whereas the chemotaxonomic methodology is good only to the Division or Class level. However, pigment analyses themselves are an objective technique whereas microscopy can be subjective in relation to the number of fields counted, bias/knowledge of the microscopist and other parameters.
Pigment-based chemotaxonomy can be extremely advantageous and cost effective in large ecosystem-scale research and monitoring programs, such as the Comprehensive Everglades Restoration Plan covering the south Florida Ecosystems. Much research remains to be done regarding the effects of light, nutrients, and their synergies on the pigment ratios we decide to use for these (chemo-)taxonomic estimations of microalgal communities. However, as this basic research continues, utilization of these methods for ecosystem surveys is ongoing. Both the basic studies as well as the monitoring efforts are active portions of the research in my laboratory.
My studies of the phytoplankton, microphytobenthos and seagrass epiphyte microalgal communities can be found on the web at http://www.aoml.noaa.gov/ocd/sferpm/louda/louda_algal_blooms.html.
Similar pigment-based chemotaxonomy studies of phytoplankton and epiphytes are being performed in the Atlantic Ocean, Florida Bay other lagoons/ estuaries, Lake Okeechobee and on periphyton in the Everglades in association with the South Florida Water Management District.
Microalgal culture:
In order to study pigment ratios of living microalgae as well as provide living cells with which to begin senescence-death studies (below), we grow pure cultures of algae, as well as purchase, beg, borrow and steal from other sources. By growing our own cells, we can provide a nutrient and light history and, even though this phase of research in its infancy here, begin to ascertain the effects of these parameters and their synergy have upon pigment quantities and ratios in various species. These data will eventually be used to ‘fine-tune’ pigment-based chemotaxonomy.
Senescence-death related alteration of pigments:
Once algal cells begin to undergo senescence and death, their pigments undergo alterations and eventual recycle. However, the fates and rates of these reactions are different not only starting structure but by the environment (oxygen fugacity, pH, light/dark, pE, etc.) to which they are exposed during these initial phases of recycle.
My interest in the senescence / death related alteration of chlorophylls and carotenoids comes from 2 separate foci.
First, hardly any natural microalgal population consists entirely of log phase perfectly “healthy” cells. That is, certain cells in senescence, dead cells, heterotrophically processed cells (fecal pellets) and even resuspended dead-older cells (sediments) can all be admixed with the healthy population. Thus, to begin to estimate the effects which these degradation processes have on the pigment ratios utilized in our chemotaxonomic estimates of the viable algae, we need information on these changes and relative rates. For example, if fucoxanthin is degraded faster than chlorophyll-a in a population of diatoms then the overall diatom chlorophyll-a contribution (to total OM, ‘living’ plus ‘dead’) will be underestimated if dead diatoms are present.
Second, my studies on the entire geochemistry continuum of chlorophyll, often including the metalloporphyrins in oils and coals, requires knowledge of the very early stages of organic diagenesis. That is, the ‘geochemical’ reactions which overlap and succeed senescence / death phenomena.
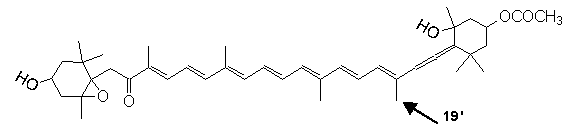
| As an example, Figure R9 is an HPLC chromatogram of a culture of the chlorophyte Closterium sp. which was aged (dark with oxygen) for 5 y8m. The phytyl esters of Mg-
purpurin-18 and chlorin-p6 were found to have been produced from Chlorophyll-a. These
pigments offer substantial proof for an early loss of the 5th (isocyclic) ring of CHLa
during death -‘diagenesis’.
These in vitro studies allow us to trace many of the reactions required or proposed to occur during ‘real world’ geochemical processing of natural pigment assemblages. Therefore, laboratory experimentation with algal cells and also with pure compounds afford us with a glimpse into the workings of nature and allow us to extend these results to the interpretation of biogeochemical processes. Here, Figure R10, the oxidation of chlorophyll-a, giving purpurin-18 can be traced to the eventual production of an “etioporphyrin”. Note the lack of the “5th” ring (see Figure R1). There are many many more experiments to do, both with microalgal cultures and/or with pure compounds. |
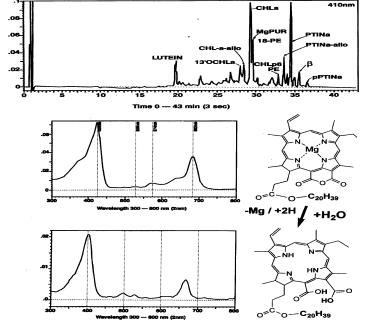 Figure R9: Generation of Purpurin-18.
|
As you may tell, my research interests span the realm of biological pigments from primary productivity / community dynamics, Everglades and coastal ecosystem restoration / monitoring, the geochemistry of pigments and relation to petroleum / coal generation, to the pure biochemistry and chemistry of the chlorophylls and carotenoids.
Return to Dr. Louda's Home Page, Chemistry Faculty Page or the Chemistry Home Page.